Scientific insights: Urea cycle disorders
Drug hunters need to watch Urea Cycle Disorders! Gene therapies (cost) and ERTs (patient experience) still leave much to be desired.
Drug hunters need to watch Urea Cycle Disorders! Gene therapies (cost) and ERTs (patient experience) still leave much to be desired.
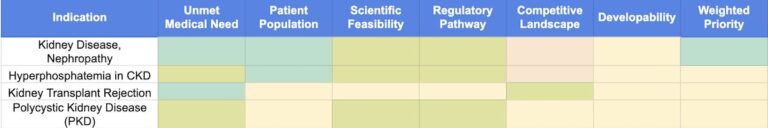
Explore why kidney disease is emerging as a top therapeutic focus. See how VibeOne identifies high-potential kidney indications and guides strategic prioritization.

AI is already transforming how leading organizations identify, evaluate, and close deals, but not all AI platforms are built for BD&L.
Fabry disease may seem niche, but it’s becoming a bellwether for how global innovation and modality shifts are reshaping rare disease pipelines.

Even with databases, access to prior knowledge in biotech BD teams often breaks down. The antidote is simple : build durable organizational memory. AI can help.

Learn how Incite partnered with Vibe Bio to accelerate sarcoma drug discovery with unbiased ranking and AI-powered compound analysis.

Learn how Orphan Therapeutics Accelerator uses Vibe Bio for rare disease due diligence, pipeline and competitive analysis, and in-licensing strategy.

Facing a flood of biopharma assets? Learn how AI can help your BD team cut through the noise, gain confidence, and act with purpose.

Learn how top biopharma BD teams find diamonds in the rough by prioritizing strategic clarity, disciplined pipeline triage, and AI-powered asset tracking.

With capital markets in flux, regulatory headwinds, and scientific risks, boards and executives must navigate pharma strategy and portfolio prioritization.