5 guardrails you MUST implement when using AI in biopharma

Implementing guardrails for artificial intelligence (AI) in the biopharmaceutical industry is crucial for ensuring the effective, safe, and compliant use of this powerful technology. While AI has the potential to transform drug development and portfolio management, it also requires domain expertise, careful oversight, and strategic implementation to maximize the benefits of AI-driven pipeline analysis while mitigating potential risks. Properly implemented and used, AI-powered pipeline analysis can help biopharma companies calculate value and evaluate risks more quickly.
The traditional role of guardrails
The concept of guardrails in biopharma isn’t new. It has evolved significantly over the years, starting in the late 1970s and focusing on production processes. Later, this expanded to encompass product design, prioritizing systematic oversight and quality control. Organizations conducted regular audits of data sources and methodologies to increase accuracy and reliability and developed standardized protocols to create consistent frameworks for data collection and analysis. Organizations also maintained documentation that tracked processes and outcomes in detail and worked to stay up to date with the latest methodological advances and industry best practices. These interconnected strategies created an ecosystem of checks and balances, or guardrails, to ensure that pharmaceutical research and development met a high standard for scientific integrity, reproducibility, and professional excellence as the industry matured.
“To create the right environment for gen AI innovation and transformation, organizations should ensure that the technology can operate safely and responsibly—with AI guardrails playing a critical role.”
– Implementing generative AI with speed and safety | McKinsey
Driven by technological advancements, regulatory changes, and evolving industry dynamics, the need for guardrails has changed over the years. For example, greater use of computers resulted in guardrails that included computer system validation and the management of electronic records. Rapidly expanding data volumes have also necessitated guardrails on data government and knowledge management, while accelerated development required organizations to adapt their guardrails to meet shorter timelines without compromising safety and efficacy standards. More recently, advanced analytics, data science, and automation have required new guardrails to ensure responsible use of these technologies. AI and machine learning (ML) are now increasingly used in drug pipeline analysis, discovery, and development, necessitating guardrails that align with ethical frameworks and governance procedures.
Manual analysis
Drug development requires time and money. Indeed, studies estimate that the research and development cost for a new drug has a wide range: anywhere from $314 million to $4.46 billion, depending on the therapeutic area, data, and modeling assumptions (using data in the United States from 2000-2018). That’s one of the biggest reasons biopharmaceutical companies need to know their market. This is called market landscaping, and it involves extensive research that explores the target market, including market size, share, trends, barriers to entry, risk factors, and the competitive environment.
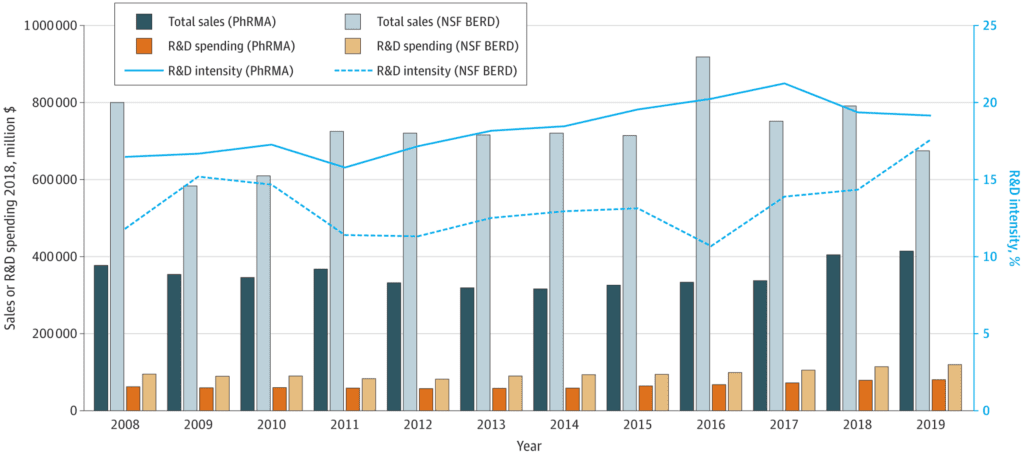
Source: Costs of Drug Development and Research and Development Intensity in the US, 2000-2018
Market landscaping takes a lot of time and human effort to incorporate scientific and clinical knowledge about different therapeutic areas, unmet patient needs, the cause of a disease and how it impacts a patient, physiology, prescribing data, competitor approaches, clinical trial data, and how well current treatment regimens are working. Human analysts use all of this information to build a detailed picture of the market for that specific therapy, including potential roadblocks to success. Companies often hire teams of analysts to do this work, which takes time and money to do effectively. Even so, there are some fairly common pitfalls, including:
- Using only U.S.-based clinical trial data
- Missing analysis of companies that are still in stealth mode
- Failing to include data from trials that went poorly
- Ignoring older data and only including the latest information
- Creating a point-in-time snapshot rather than keeping up to date with the latest information
Even when biopharma consultants are aware of and avoid these common issues, the sheer volume and variety of data sources today make it extremely difficult to quickly deliver comprehensive, accurate results.
AI-powered analysis with guardrails can help
AI is great at processing large datasets, making predictions, and improving decision-making, but the results are unreliable without guardrails. Guardrails play an important role in ensuring the responsible and effective implementation of AI technologies in the pharmaceutical industry. Here are five guardrails necessary when using AI-powered drug pipeline analysis:
- Scientific integrity — In order to meet high standards of scientific integrity, AI must have guardrails that ensure data quality and relevance
- Regulatory compliance — To meet the complex requirements of a wide range of industry standards, AI must have guardrails that ensure regulatory compliance
- Risk mitigation — In order to prevent inherited biases from existing datasets from compromising the decision-making process, AI must have guardrails that eliminate these biases
- Human expertise and judgment — To conduct pharmaceutical research accurately and ethically, AI must have guardrails in place that incorporate human expertise in the analysis and decision-making process
- Reliable, consistent outputs — For researchers, clinicians, and regulatory bodies to trust the results of AI-powered analysis, the AI solution must include guardrails that ensure reliable and consistent outputs
By establishing these five guardrails, biopharmaceutical organizations can leverage AI’s potential to accelerate drug pipeline analysis while protecting the fundamental principles of patient safety, scientific rigor, and ethical research practices.
AI can simplify guardrail enforcement
In drug pipeline analysis, AI can actually make the enforcement of guardrails much easier. For example, you can use AI to:
- Ingest and normalize structured and unstructured data
- Automate data validation
- Apply cleansing algorithms to ensure the data is clean
- Detect anomalies in data sets more easily
- Enable version control and audit trails for AI models
- Use explainable AI techniques for transparency in decision-making
- Allow continuous monitoring and feedback loops for AI performance
As AI models become more complex over time, guardrails can help maintain transparent and explainable decision-making processes.
AI drug pipeline analysis
With the right guardrails in place, AI can play an important role in drug pipeline analysis. For example, AI can improve portfolio management in pharma, provided biopharma experts are involved in defining clear objectives and ensuring the integration of multiple data sources. And while AI can simplify the process of calculating and comparing the value and risks of pursuing different drug candidates, it’s also important to update these analyses regularly, with humans making the final decision about which drug candidates to pursue.
AI can also improve the risk assessment process by making it easier and faster to analyze vast amounts of historical data to identify potential risks while also making it simpler to monitor emerging risks in real-time. Using an AI-powered pipeline analysis platform, it becomes far simpler to simulate a number of scenarios to quantify the potential impacts of different scenarios, with human experts providing the scenarios to test. This helps organizations to make data-driven decisions about different risk mitigation strategies more readily.
AI-powered analysis can also help with analyzing the market landscape, provided a biopharma expert clearly defines the scope and objectives of the analysis, uses diverse and reliable data sources, and implements cross-validation of AI-generated insights. The AI model must be regularly updated and refined to use new market data to ensure that it uses the latest information available.
Best practices for using AI in biopharma
- Clearly define goals and desired outcomes before deploying AI
- Implement rigorous data validation processes to prevent “garbage in, garbage out” scenarios
- Recognize that humans are critical to AI analysis, particularly in terms of deciding what to input into AI systems and interpreting outputs
- Integrate AI analysis with regulatory, market, and business insights
- Implement processes to ask questions multiple ways or cross-verify AI-generated responses for consistency
- Be aware of what AI can and cannot do in your specific context
- Conduct periodic audits of AI systems and their outputs to ensure continued reliability and relevance
By implementing these guardrails, pharmaceutical companies can harness the power of AI while maintaining the integrity, safety, and efficacy of their processes. Remember, AI is a tool to improve human decision-making, not replace it. The key lies in striking the right balance between technological innovation and human expertise to drive meaningful advancements in pharmaceutical research and development to save lives and improve health outcomes.
